Nuberg Technology & Innovation Centre
Hydrogen Peroxide Technology
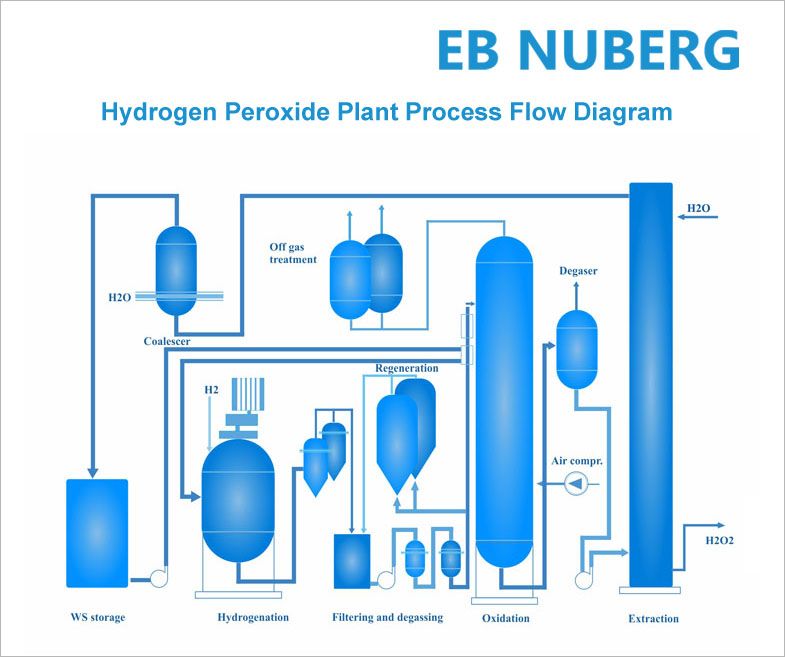
Hydrogen Peroxide (H2O2) is manufactured by anthraquinone process. In Nuberg Technology & Innovation Centre’s patented hydrogen peroxide process methodology; the chemical is manufactured by the circulation of an organic working solution (consisting of different solvents and 2-ethyl anthraquinone, which is the active chemical). The working solution is hydrogenated and oxidized, after which the product is extracted using very pure water.
Hydrogenation
Hydrogen gas is added to the working solution in the presence of a Pd metal catalyst. The hydrogen is chemically bonded to the 2-ethyl anthraquinone. The catalyst is filtered off and returned to the hydrogenation reactor.
Oxidation
When the working solution is contacted with compressed air the oxygen of the air reacts with the hydrogen that has been bonded to the 2-ethyl anthraquinone. This reaction forms the hydrogen peroxide, which is dissolved in the working solution.
Extraction
To get the H2O2 in a useable form it is extracted from the working solution using a very pure water. The product is a hydrogen peroxide solution with a concentration of typically 35 to 40 weight percent. This can be further concentrated by distillation, for example if the H2O2 is to be transported to an end user. The peroxide free working solution is returned to the hydrogenator to start a new cycle.
Special features of Nuberg Technology & Innovation Centre’s Advanced technology:
- The Working Solution is based on TBU, tetrabutylurea, giving high degree of hydrogenation and low flow of WS in the plant.
- The hydrogenation reactor is a slurry reactor with mechanical agitation and gas re-cycling, giving low hydrogen consumption, low catalyst inventory, low catalyst consumption, high reaction selectivity (=low formation of side products) and smooth operation.
- Counter-current oxidation with internal cooling, giving short hold-up time, low compressed air consumption and low by-product formation.
- High hydrogen peroxide concentration (typically 38 to 40%) extracted from the extraction column, giving low steam consumption in the following distillation plant were the concentration is increased to 50%. The distillation unit is equipped with vapor re-compression for further reduction of steam consumption.
- Very low effluent levels to water and air.
Low costs of production
| Raw materials and Utilities | Consumption/ton H2O2 | Consumption/ton product 50% conc. |
|---|---|---|
| Hydrogen, Nm3 | 710 | 355 |
| Steam, ton | 1.2 | 0.6 |
| Electricity, kWh | 800 | 400 |
| 2-EAQ, kg | 0.8 | 0.4 |
| TBU, kg | 0.5 | 0.25 |
| Aromatic Solvent, kg | 5 | 2.5 |
| Alumina, Al2O3 | 10 | 5 |
| Catalyst (2% Pd), gram | 25 | 12.5 |
| Nitrogen, Nm3 | 40 | 20 |
| Phosphoric Acid, kg | 1.0 | 0.5 |
| Stabilizer, kg | 0.4 | 0.2 |
